
Question #1: Biology
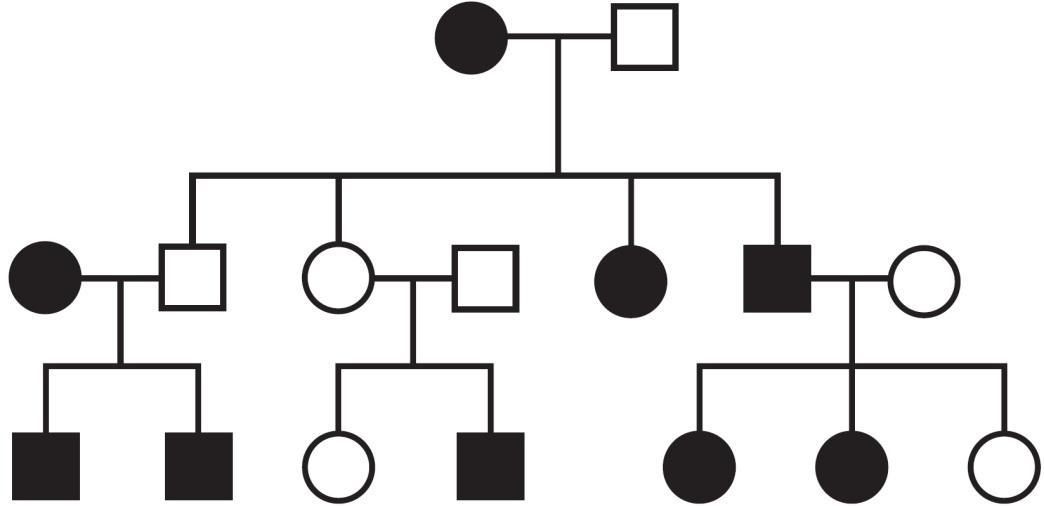
What is the inheritance pattern of the observed trait indicated by the pedigree below?
A. Autosomal recessive
B. Autosomal dominant
C. X-linked recessive
D. Cannot be determined
Answer:
(A) "Autosomal recessive" is correct.
(A) "Autosomal recessive" is correct.
Pedigrees show the distribution of a single observable trait, or phenotype, across a family tree. In classical genetics, each phenotype is determined by a combination of two alleles contributed by two copies of the same (but not necessarily identical) chromosome. One allele is generally dominant, meaning it is expressed if it is present at all. In contrast, the other allele is recessive, meaning it is only expressed in the absence of a dominant allele, which generally means two copies need to be inherited to display the recessive phenotype. The exceptions are those alleles found on the X chromosome in males; males' sex chromosomes include only one X (and one Y), so each trait coded for on the X chromosome is determined by only one allele instead of a combination of two alleles. This means it's statistically more likely for males to inherit recessive X-linked traits since only one copy of the recessive alleles needs to be inherited to display the recessive phenotypes, as opposed to the usual two.
The fastest way to determine which inheritance pattern is shown by a pedigree, then, is to use the Kaplan shortcut: Identify whether two matching parents have an opposite offspring. If two affected parents have an unaffected offspring, both parents must have been heterozygous (having one of each allele), and the trait must be dominant: Rr Rr rr. If two unaffected parents have an affected offspring, both parents must have one again been heterozygous, but in that situation, the trait being tracked must have been the recessive one: Rr Rr rr. In the pedigree provided in this question, generational skipping occurs in the middle portion: Generation 2 has two unaffected parents, but generation 3 has an affected offspring. This indicates a recessive trait. Since a roughly equal number of males and females are affected (5:4 ratio), this is an autosomal trait, and A is the correct answer.
Question #2: General Chemistry
What is the oxidation state of each nickel on the reactant side of the following reaction?
2 NiO(OH) + Cd + 2 H2O 2 Ni(OH)2 + Cd(OH)2
A. -2
B. -1
C. +1
D. +3
Answer:
(D) is correct.
(D) is correct.
Oxidation numbers provide a way to keep track of the movement of electrons in a reaction. Several rules govern how oxidation numbers are calculated, but in this situation, it's only important to recognize the oxidation numbers of the common components attached to nickel [Ni] on the reactant side and to remember that the sum of the internal oxidation numbers of a molecule equals that molecule's net charge. In this case, nickel oxide [NiO(OH)] is neutral, so the sum of its internal oxidation numbers must be 0. Hydroxide [OH–] always has an oxidation number of -1, and oxygen [O] almost always has an oxidation number of -2. To make these charges cancel out to equal 0, the oxidation number of nickel [Ni] must be +3: 3 - 1 - 2 = 0. D is the correct answer.
Question #3: Organic Chemistry
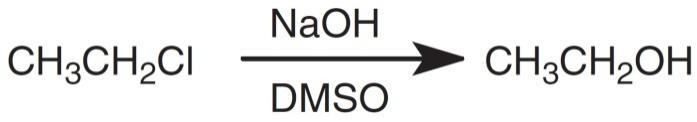
Given the following reaction conditions, which statement is most accurate?
A. The reaction follows first-order kinetics and is a concerted reaction.
B. The reaction follows first-order kinetics and involves formation of a carbocation.
C. The reaction follows second-order kinetics and is concerted.
D. The reaction follows second-order kinetics and involves formation of a carbocation.
Answer:
(C) is correct.
(C) is correct.
This substitution reaction shows chloride (Cl–) being replaced by hydroxide (OH–). Substitution reactions occur mainly via one of two mechanisms: SN1 (unimolecular kinetics and two steps with a carbocation intermediate) or SN2 (biomolecular kinetics and one step). In this situation, the mechanism isn't shown but can be inferred based on the reaction conditions. First, the reactant with the carbons (the substrate) has a strong leaving group; the Cl– that detaches is relatively stable as an ion in solution by itself (think of how table salt, NaCl, is able to readily dissolve into Na+ and Cl– in water). Second, the substrate has primary substitution, meaning the carbon attached to the leaving group is only attached to one other carbon, which in turn means that there is little steric hindrance but also that the carbon doesn't have many other carbons to stabilize it if it were to gain a charge. Third, the other reactant, OH–, is a strong base and strong nucleophile, indicating that it can readily attack the substrate on its own. Finally, the solvent DMSO (dimethyl sulfoxide) is polar aprotic so can stabilize the leaving group without deactivating the nucleophile. All of these factors point toward an SN2 reaction. Since an SN2 reactions is always concerted, occurring in one step without forming discrete intermediates, the correct answer is C.
Question #4: Organic Chemistry
(R)-1-Fluoro-1-iodopropane is reacted with NaN3 in HMPA. Which of the following is the major product of this reaction?
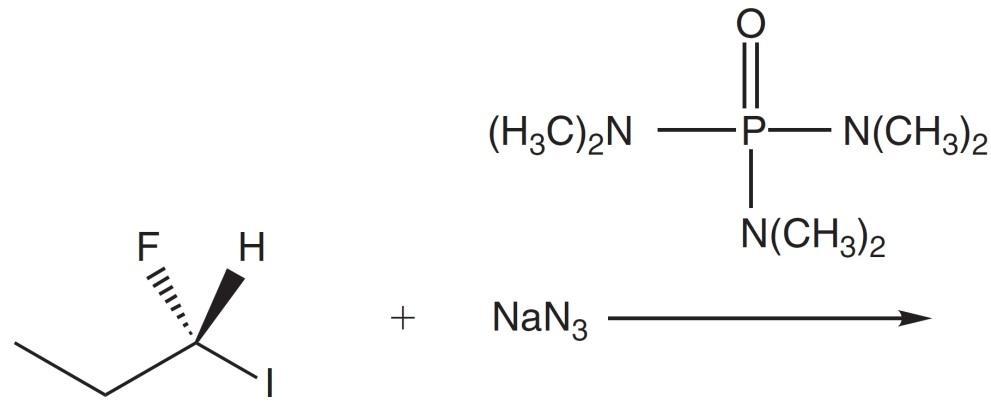
A. (R)-1-Azido-1-fluoropropane
B. (R)-1-Azido-1-iodopropane
C. (S)-1-Azido-1-fluoropropane
D. (S)-1-Azido-1-iodopropane
Answer:
(A) is correct.
(A) is correct.
Although the reactants may look complicated, this reaction is actually just a classic SN2 reaction. Azide (N3–) is the nucleophile, and iodide (I–) is the leaving group. Note that, although fluorine is a halogen, fluoride (F–) does not follow the group trend and is not a good leaving group due to its small size. With that substitution in mind, answer choices B and D can be eliminated.
However, SN2 reactions are stereoselective with an inversion of stereochemistry and therefore only create one of the two possible R-S isomers. Since the leaving group and the nucleophile end up having different priorities, the absolute configuration (R vs. S) must be recalculated. Start by drawing out the new molecule (remembering to invert the stereochemistry, like turning an umbrella inside out) and ranking the attached atoms in order of decreasing atom mass: F > N > C > H. From there, connect 1 2 3 with an arrow. The arrow is counterclockwise, initially indicating an S configuration. Finally, check atom 4: If it is into the page, leave the configuration how it is; if it is coming out of the page, reverse the configuration. In this case, H is coming out of the page, so the final configuration is R, and the correct answer is A.
Fill out the information below to get the answers and explanations. We’ll also email you free tips, admissions advice, exclusive offers, and other content aimed specifically to help you do better on Test Day.